Artificial Intelligence and Machine Learning in Clinical Trials Dissertation
This study will explain how Artificial Intelligence (AI) and Machine Learning (ML) could improve clinical trials in the pharmaceutical industry. The contents of this paper will give details on how the two technologies could help advance the efficacy of clinical trials using data and statistics generated from companies that have adopted them. At the same time, to draw contrasts on the application of AI and ML in the health sector, the limitations of the technologies will also be elucidated to highlight areas of improvement that could be explored for future improvements and integration in clinical practice. To understand these areas of research in detail, in this analysis, emphasis will be made to highlight the role of the technology in addressing poignant challenges associated with clinical trials, such as the recruitment and selection of participants. This area of the investigation will encompass most of the analysis included in the present text but the role of AI in optimizing dosing regimens and improving the design of effective interventions will also be explored as supplementary analyses. Before embarking on these areas of analysis, it is important to understand some of the most significant challenges clinicians experience when completing their trials.
We will write a custom essay on your topic
810writers online
Cost and Time Associated with Clinical Trials
Recruiting patients for clinical trials is marred by challenges relating to the incompletion of tests and rising costs of sustaining volunteers throughout the investigation. This is why pharmaceutical companies pay a lot of money in research and development before they develop and present new drugs to the market (11). The costs associated with developing such drugs could stretch into millions or even billions of dollars, depending on the design and site selection protocol of adhering to clinical research guidelines (10). These findings mean that pharmaceutical companies have to invest many resources in minimizing the time taken to conduct clinical research and present safe drugs to the market. Part of the process involves making sure that a participant who is willing to engage in a clinical research trial is committed to staying in a program for its full length. However, clinicians do not always achieve this objective because of the high failure rates associated with past assessments and the costs of keeping the participants engaged throughout different stages or phases of clinical research (11). Figure 1 below shows the average cost of maintaining a volunteer, across different phases of a typical clinical research trial.
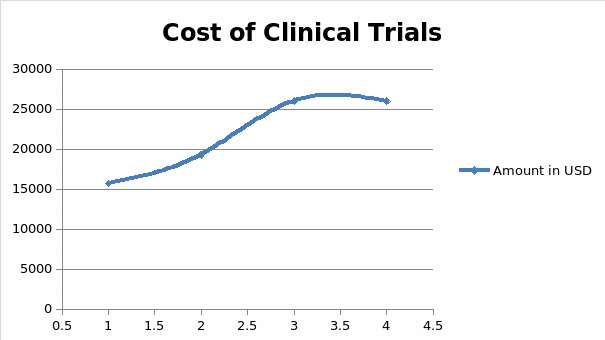
Figure 1. Cost of clinical trials (Adapted from Roth 11)
According to figure 1 highlighted above, the cost of maintaining the participation of research participants rises across four phases of clinical trial development. On average, the first phase of clinical research could cost pharmaceutical companies up to $15,700 to maintain one participant in the first phase of drug development. In the second stage, this cost could rise further to $19,300, after which it later increases to $26,000 in the third and final stages of the clinical trial. For most drug developers these costs are often prohibitive and inhibit their ability to develop reliable drugs and present them to the market affordably (11). This is why the cost of some drugs is often high and unreachable to patients who need them the most (10). High rates of incomplete clinical trials, reported in some cases, further compound the problem. Figure 2 below shows that researchers fail to complete about 80% of clinical trials fail on time, while another 20% are delayed for more than six months due to reasons attributed to recruitment and retention of patients in these investigations (11).
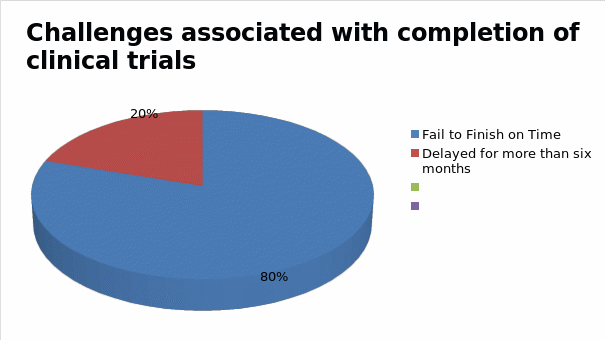
Figure 2. Challenges associated with completion of clinical trials (Source: Adapted from the works of Roth 11)
The findings highlighted above show that many pharmaceutical companies are experiencing challenges maintaining a healthy balance between the overall cost of conducting clinical research and the time it takes to complete them. This problem has affected clinicians across different areas of research (10). Consequently, there is a need to find better ways of managing these variables. AI and ML provide opportunities for doing so as highlighted below.
Bolstering Patient Recruitment Efforts
As mentioned above, recruitment
Our Advantages
Quality Work
Unlimited Revisions
Affordable Pricing
24/7 Support
Fast Delivery